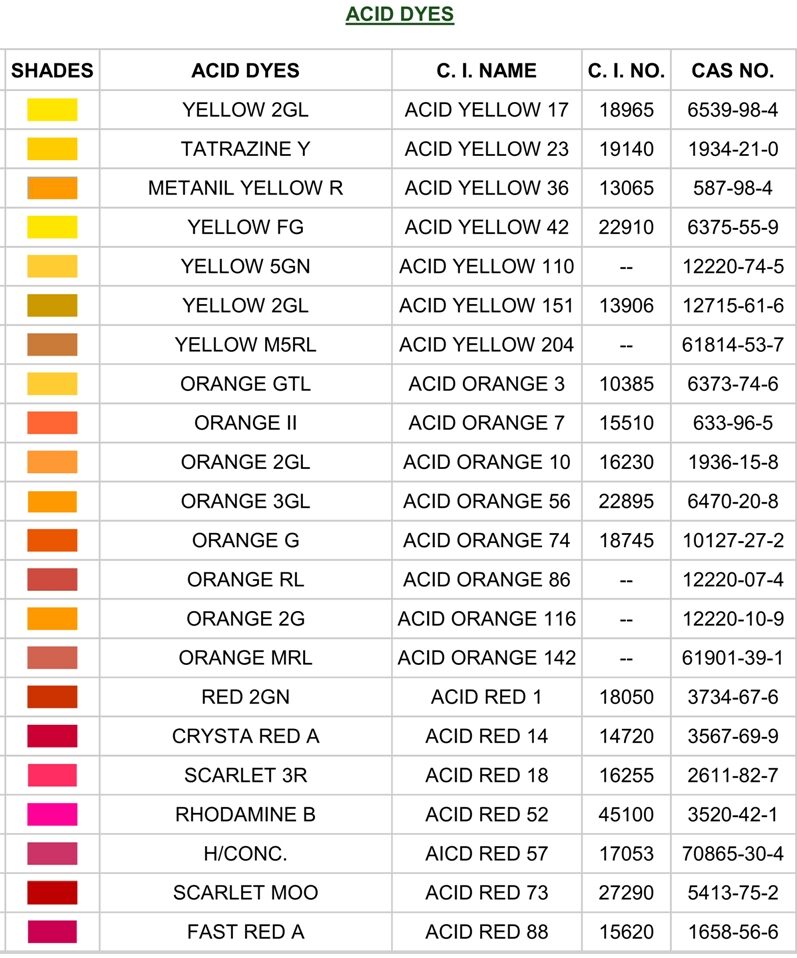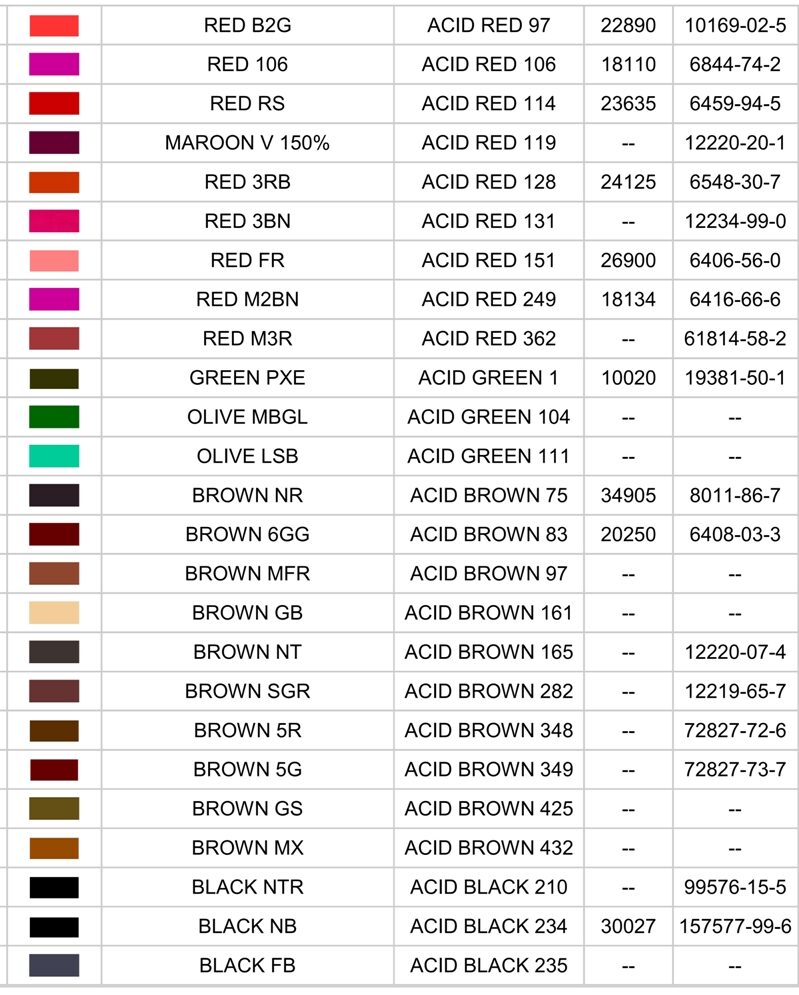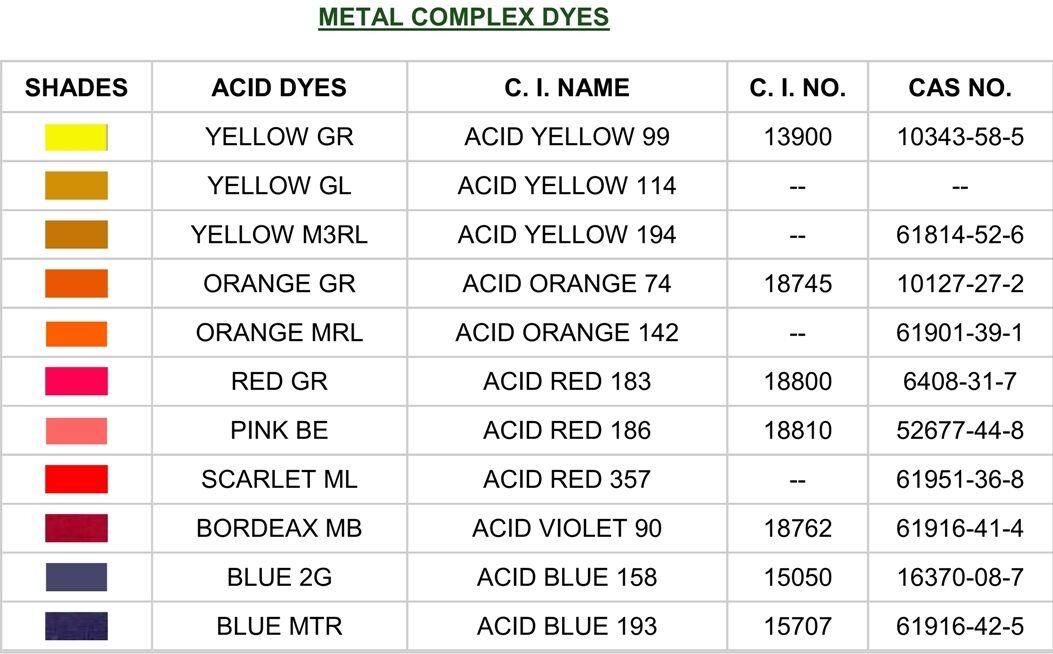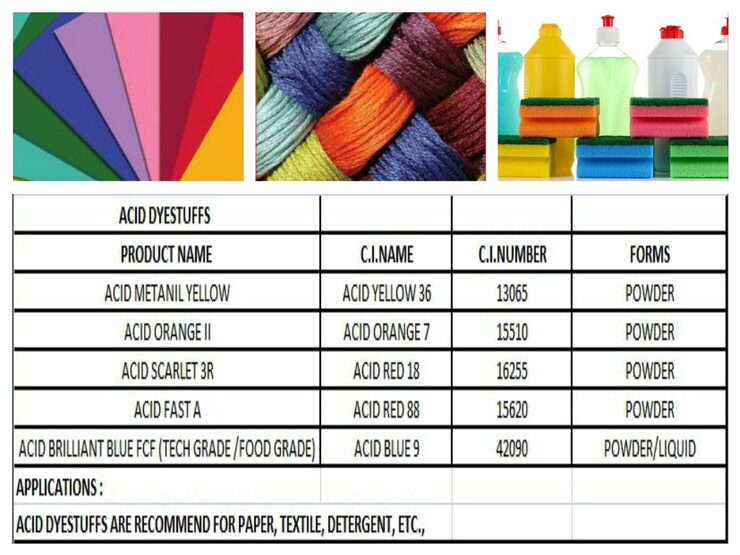
Acid dyes are a type of synthetic dye that is water-soluble and is primarily used to dye protein fibers such as wool, silk, leather, paper, ink and nylon. These dyes are called "acid" dyes because they are typically applied in an acidic dye bath, where the presence of an acid helps the dye molecules to bond with the fibers.
Acid dyes are characterized by their vibrant and intense colors, making them popular for dyeing textiles, yarns, and fabrics. They can produce a wide range of colors, including bright and deep shades. Additionally, acid dyes are known for their excellent lightfastness and washfastness, meaning that the colors remain stable when exposed to light and washing.
The dyeing process with acid dyes usually involves dissolving the dye in hot water along with an acid, such as acetic acid or sulfuric acid, to create the dye bath. The material to be dyed is then immersed in the dye bath, where the dye molecules penetrate the fiber and form bonds with it. The dyeing process may also involve additional steps such as heating, agitation, and rinsing to ensure proper dye uptake and fixation.
Overall, acid dyes are widely used in the textile industry for their versatility, colorfastness, and ability to produce vibrant colors on protein-based fibers.