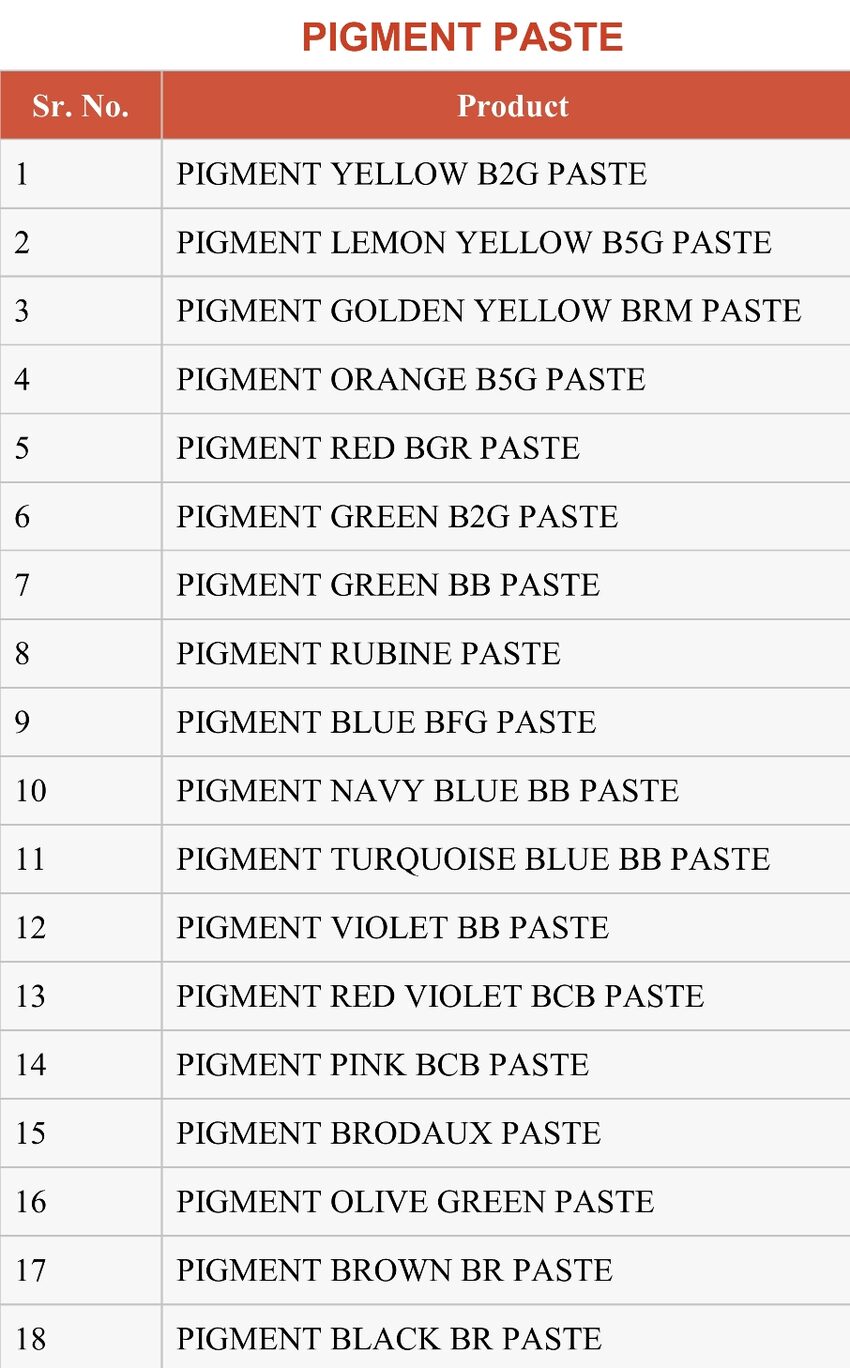
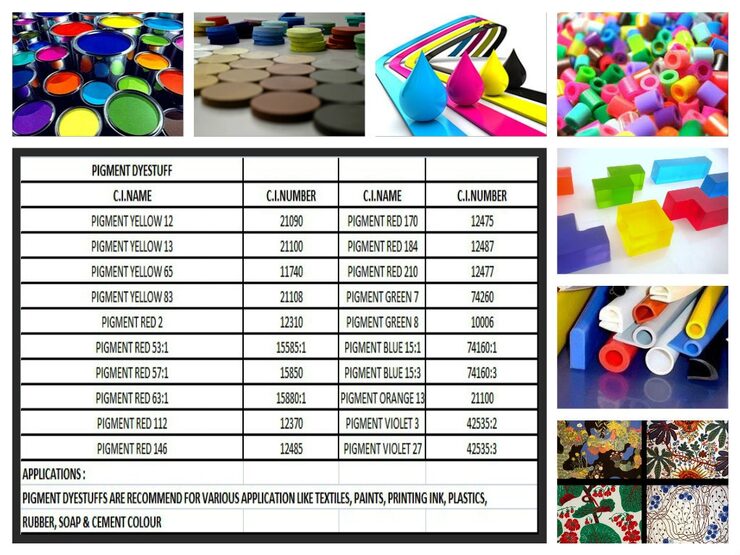
Pigments are finely ground, insoluble solid particles that impart color to various materials through dispersion. Unlike dyes, which dissolve and penetrate the material they color, pigments remain suspended in the medium, forming a dispersed phase. Pigments are widely used in a variety of applications, including paints, inks, plastics, cosmetics, and ceramics.
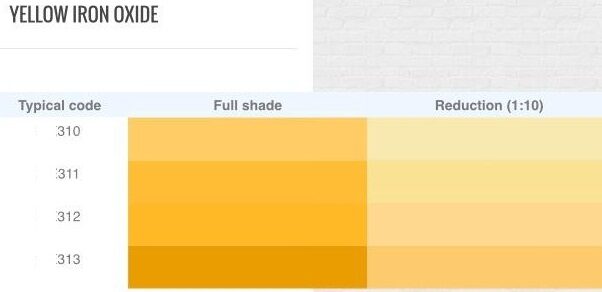
Iron oxides are chemical compounds composed of iron and oxygen atoms. They are commonly found in nature as minerals and are widely used as pigments in various applications due to their natural abundance, stability, and range of colors.
The most common types of iron oxides include:
-
Iron(II) oxide (FeO): Also known as ferrous oxide, this compound is black in color and is commonly found in the form of the mineral wüstite. It is less commonly used as a pigment compared to other iron oxides.
-
Iron(III) oxide (Fe2O3): This compound exists in several forms, including hematite (red), magnetite (black), and maghemite (brown). Hematite is the most common form used as a pigment, providing red and brown colors. Magnetite is often used in magnetic recording media and as a black pigment. Maghemite is less common but can also be used as a pigment.
Iron oxides are widely used as pigments in various applications, including:
-
Paints and coatings: Iron oxide pigments are extensively used in paints and coatings due to their excellent color stability, durability, and UV resistance. They are commonly used in exterior coatings for buildings, bridges, and automotive applications.
-
Plastics: Iron oxide pigments are used in plastics to impart color and opacity. They are commonly used in products such as plastic packaging, toys, and automotive parts.
-
Construction materials: Iron oxide pigments are added to concrete, mortar, and paving materials to provide color and enhance the aesthetic appeal of structures such as buildings, roads, and sidewalks.
-
Inks and pigmented materials: Iron oxide pigments are used in printing inks, toners, and pigmented materials such as ceramics, paper, and textiles.
Iron oxide pigments are valued for their natural earthy colors, lightfastness, chemical stability, and resistance to heat and weathering. They are produced through various methods, including natural extraction from mineral deposits and synthetic processes such as precipitation and thermal decomposition.
Overall, iron oxides play a crucial role as pigments in numerous industries, providing coloration and enhancing the visual appearance and performance of a wide range of products and materials.